Categories
Select a new category
Largest Study to Date Finds Autism Is Mostly Heritable
By Chelsea Toledo, M.A. on August 15, 2019
Background: Autism Spectrum Disorder (ASD) can be caused by multiple risk factors. Risk factors increase the likelihood of developing a condition and can be genetic or environmental. Genetic risk factors relate to variations in our DNA, while environmental factors stem from social, physical, or chemical exposures and can be present during fetal development or months to years after birth.
What’s New: A recent study set out to determine the nature of the risk factors that were most commonly associated with an ASD diagnosis. The authors analyzed the largest-ever dataset for this purpose – containing medical information for more than 2 million births from five different countries: Denmark, Finland, Sweden, Israel, and Western Australia.
Looking at multiple generations represented within the dataset, the researchers found:
- In total, 22,156 ASD diagnoses were recorded within the sample (about 1 percent).
- Overall, more than 80 percent of ASD cases were found to be heritable.
- Heritability varied by country, ranging from 50.9 percent heritability in Finland to 86.8 percent heritability in Israel.
- Maternal effects (such as illness during pregnancy) were associated with only about 1 percent of causes.
- Other environmental risk factors were found to have an additive effect with genetics.
Why it’s important: This is the largest-ever study to suggest that ASD is largely genetic. As this study suggests, environmental factors interact with genetic risk factors and their association with ASD varies by country – a pattern that merits future study.
Help me understand :
| Source(s) : |
| Tweet |
Autism Linked to Disproportionate Gene Inheritance
By Chelsea E. Toledo, M.A. and Sharmila Banerjee-Basu, Ph.D. on June 30, 2017
Background: Autism spectrum disorder, or ASD, is highly heritable. For instance, children who have a sibling with an ASD diagnosis are far more likely to be diagnosed with the disorder themselves than children whose siblings don’t have ASD. While that trend and other findings have pointed to genetic risk factors contributing to ASD, it is not yet clear how the inheritance and expression of genes leads to the disorder.
What’s New: On May 15, 2017, the journal Nature Genetics published a study exploring genetic architecture underlying ASD. In this study, the authors analyzed data from 6,454 families with at least one child with a diagnosis of ASD. The team of scientists calculated common polygenic risk for ASD, educational attainment, and schizophrenia for all genotyped family members. They found that polygenic risk—variations in multiple genes associated with the condition—was significantly over-transmitted to affected children but not to unaffected siblings. Moreover, the common polygenic variants contributed to ASD risk even in children with damaging autism-associated genetic change that is not present in either parent.
Why it’s important: This study suggests that autism risk is additive. Both common and rare variants comprise the genetic architecture in ASD. Children with ASD also over-inherited genetic variants related to schizophrenia and educational attainment indicating their positive association.
Help me understand :
| Source(s) : |
| Tweet |
New Genetics Study Finds Autism-Innate Immunity Link
By Shana R. Spindler, Ph.D. on January 18, 2017

Background: Have you ever used one object in two ways? For example, you can use a spatula to flip an egg or swat a fly. The cells in our bodies do this all the time with the genes in our DNA. If something goes wrong with a gene, several problems in the body can occur. Think about the spatula. If the spatula factory accidentally shapes the flat surface like a spoon, you’ll have a hard time flipping eggs or smashing a fly. These are different activities, but the misshapen spatula affects both.
To learn more about the genetics behind autism spectrum disorder (ASD), researchers are looking at co-occurring symptoms that happen more frequently with autism than in the general population, such as seizures, infections, gastro-intestinal disorders, heart problems, and psychiatric disorders. The researchers hypothesize that these activities in the body may share a common gene or set of genes that, when disrupted, increase risk for ASD core symptoms and co-occurring symptoms at once.
What’s new: Researchers from Harvard Medical School and the Massachusetts Institute of Technology made an important link between ASD and the immune system by combining genetic data collected during other studies. Using a series of statistics calculations, the researchers found that genes involved in innate immunity were most likely to have problems across co-occurring conditions with ASD.
Innate immunity is your first line of defense to foreign invaders, like harmful bacteria. The researchers narrowed in on two parts of innate immunity that appear to be most significant. One is a group of proteins, called toll-like receptors, which help the body recognize microbes. The second includes chemokines, small signaling molecules that help attract nearby immune system cells.
Why it’s important: These results suggest a path forward to look at the genetic and environmental interactions behind some cases of autism. A common mechanism causing distinct symptoms may also aid in the development of targeted ASD therapies.
Help me understand :
| Source(s) : |
| Tweet |
Four Key Trends Found for ASD Traits and Recurrence
By Shana R. Spindler, Ph.D. on November 5, 2015

Background: Several studies suggest that in families containing one or more individuals with Autism Spectrum Disorder (ASD), non-diagnosed members have increased presence of ASD-related traits. However, little is known about the prevalence or nature of these traits among the siblings in families where multiple members have ASD.
What’s new: On October 27, 2015, the journal Molecular Autism published a study exploring autism symptom pattern and recurrence in families with children on the spectrum. The researchers compared data for over 5500 siblings from the Autism Interactive Network. By examining single versus multiple incidence of ASD in a family, as well as the affected gender, the researchers discovered four key trends:
- Non-diagnosed children who have more than one sibling with autism possess an increased and specific pattern of autistic traits—namely resistance to change and restricted interests.
- Children with ASD from multiple incidence families are less symptomatic than those from single incidence families.
- A history of language delay with atypical speech is a risk factor for both social and restrictive/repetitive behavior symptoms in children not diagnosed with ASD from single or multiple incidence families.
- Males who are born into a multiple incidence family including at least one female with ASD are at a greater risk for possessing autistic traits. Likewise, families containing any number of females with ASD have greater recurrence risk for future children.
Why it’s important: This study may useful for the genetic counseling of families with ASD who are considering having another child, or for parents who are worried about their children’s risk of having a child with ASD. The study supports the idea that females require a greater risk factor burden before entering into an ASD diagnosis, making it more likely that males in the same family will have more severe autistic traits. It’s important to note, though, that currently there is no way to predict if anyone will or will not have a child with ASD or ASD-related traits.
Help me understand :
| Source(s) : |
| Tweet |
Combined Genetic Tests May Improve ASD Management
By Chelsea E. Toledo, M.A. on September 24, 2015

Background: Autism spectrum disorder (ASD) is thought to be caused by a combination of genetic and environmental factors. To date, variations in hundreds of genes have been associated with ASD, and these explain only a small fraction of individuals with autism. Although no genetic tests are available for the diagnosis of ASD, genetic testing could offer guidance for medical management. Some tests look for big changes in DNA, while others check for small variations.
What’s New: On September 1, 2015, the Journal of the American Medical Association published a study exploring the utility of combining two genetic tests. The researchers administered chromosomal microarray analysis (CMA) – which identifies large genetic abnormalities, such chunks of missing DNA – to 258 unrelated children with an ASD diagnosis. They then applied whole-exome sequencing (WES) – which helps to identify variations in the protein encoding portions of DNA – to 95 of those children, who also had physical abnormalities sometimes associated with the disorder. They found that, individually, those tests could reveal information about a child’s susceptibility to ASD about eight percent of the time. However, when applied together in children with physical abnormalities, CMA and WES were able to provide an informative diagnosis 38 percent of the time.
Why it’s important: While still not precise enough to provide an initial ASD diagnosis, genetic tests can be useful in informing ASD medical management. Autism-linked genetic variations might predispose a child to other medical conditions, and when combined, CMA and WES could help care providers identify what comorbid conditions to prepare for.
Help me understand :
| Source(s) : |
| Tweet |
Study Links Autism to Congenital Abnormalities
By Chelsea E. Toledo, M.A. on June 18, 2015

Background: Autism Spectrum Disorder (ASD) is characterized by differences in behavior, communication, and social interaction and has been linked to genetic and environmental risk factors. Studies haves suggested that congenital abnormalities such as cleft lips and palates—which begin to form early in pregnancy—are more common in children with ASD.
What’s New: On June 3, 2015, the Journal of Autism and Developmental Disorders published a study exploring the relationship between various congenital abnormalities and ASD in children with and without intellectual disabilities. The researchers examined the records of 17,695 Finnish children born between 1987 and 2000—4,441 with ASD and 934 with congenital abnormalities. They found that children with ASD were more likely to have congenital abnormalities of the eye, face, and neck, as well as the central nervous and musculoskeletal systems, development of which occurs during the first trimester. They also found that both the children with ASD and those with congenital abnormalities were more likely to have been born prematurely or at a low birth weight.
Why it’s important: This study suggests that—while ASD isn’t typically diagnosed until children are at least two years old—the underlying factors leading to the disorder may appear very early during gestation. Further research could illuminate the precise environmental, genetic, and epigenetic influences leading to ASD in the developing brain.
Help me understand :
| Source(s) : |
| Tweet |
Twin Study Finds Autism Risk Is Largely Genetic
By Chelsea E. Toledo, M.A. on March 11, 2015

Background: Autism Spectrum Disorder (ASD) affects about 1 percent children worldwide. Many children who are diagnosed have a sibling with ASD. Research has suggested that the disorder is heritable. While some researchers report that genetic factors are more predictive of ASD, other investigations suggest that environmental factors play an equal, if not larger, role.
What’s new: On March 4, 2015, the journal JAMA Psychiatry published a study of ASD in pairs of twins. The researchers recruited participants of a longitudinal study on identical twins, who share the same DNA, and fraternal twins, who have DNA similarity equivalent to non-twin siblings. After an initial screening of more than 6,000 twin pairs born in the United Kingdom between 1994 and 1996, the researchers conducted additional evaluations for ASD on smaller groups of twin pairs. They found that the identical twins were far more likely to share an ASD diagnosis. They also found that some environmental factors, such as living in an area with high air pollution, increased the risk of an ASD diagnosis—but that those played less of a role than genetic influences.
Why it’s important: Using a large cohort of twin pairs and a variety of diagnostic tools, the researchers have highlighted the genetic underpinning of ASD and ASD-related traits. Furthermore, they showed that the liability to ASD resides mainly in the additive effects of genetic factors. Future studies could probe genetic and environmental factors further—examining the predominance of ASD in boys and exploring possible interactions between genetics and the environment, for instance.
Help me understand :
| Source(s) : |
| Tweet |
Inflammation-Related Genes Ramped Up in ASD
By Chelsea E. Toledo, M.A. on December 23, 2014

Background: The root cause, or causes, of Autism Spectrum Disorder (ASD) remains unknown. Many studies point to a genetic component and have uncovered genetic glitches that appear in people with ASD. To understand how or whether those genes are expressed as traits, however, scientists would have to look for effects in the brain itself.
What’s new: On December 10, 2014, the journal Nature Communications published a large study on gene expression in the brains of people with ASD. The researchers examined 104 tissue samples from a total of 72 autopsied brains—47 from people with ASD and 57 controls with typical development. They found significant differences in gene expression within microglial cells, which respond to infectious agents and other threats that lead to inflammation in the brain. In the brains of people with ASD, they found that microglial cells were perpetually active, with genes for inflammation response turned on—likely a response to processes related to autism, and not the other way around.
Why it’s important: This is the largest data set examined to date on gene expression related to autism. Future studies could solidify whether treating inflammation could improve ASD symptoms.
Help me understand :
| Source(s) : |
| Tweet |
Updated Catalog of Autism Genes Unveiled
By Sharmila Banerjee-Basu, PhD on November 4, 2014
Background: Autism Spectrum Disorder (ASD) is a genetically heterogeneous condition in which hundreds of genes have been associated with the disorder. Large consortiums of scientists are working together to better define the genetic underpinnings of autism. An unmet challenge in the field is how to connect the genetic profile to clinical features of ASD—a necessary first step for targeted treatment.
What’s new: Two research groups report an updated list of ASD risk genes based on statistical analysis that quantifies the genes’ role in the October 29, 2014, online issue of Nature. Both groups utilize exome sequencing—where scientists read only the protein coding part of the genome—to sequence the DNA of individuals with ASD and controls. The larger study, led by an International Consortium, sequenced 3,871 ASD cases and 9,937 controls. The other study analyzed more than 2,500 families with a single child affected by ASD from Simons Simplex Collection. Together, the studies reveal an updated list of ASD-associated genes, including several novel genes.
Why it’s important: The new studies have advanced our understanding of autism by replicating earlier findings of excess, spontaneously arising genetic glitches—known as de novo mutations—in ASD individuals. Another key finding addresses the gender bias in autism: ASD females and ASD males with low IQ have a different genetic risk architecture than ASD males with high IQ. Finally, the risk genes belonging to individuals with severe ASD significantly overlap with risk genes identified in Schizophrenia and Intellectual disability.
Help me understand :
| Source(s) : |
| Tweet |
Common DNA Variations May Be Largest Factor in Autism
By Shana R. Spindler, Ph.D. on July 22, 2014

Background: The list of autism-associated genes has grown dramatically as researchers identify more and more genetic risk factors. But are these autism-linked genetic variations more often inherited from parents or formed de novo in the child?
What's new: Common, inherited variations in DNA may be the largest cause of autism, according to a new study published in Nature Genetics. According to the researchers, a little over 50 percent of autism cases are from a combination of widespread genetic variations, which alone do not lead to autism, passed from parents to a child. In contrast, new DNA mutations in the child only account for 2.6 percent of autism cases.
Why it's important: This study highlights the complexity of common genetic variations underlying autism.
Help me understand :
| Source(s) : |
| Tweet |
Elevated Womb Hormone Levels Linked to ASD
By Chelsea E. Toledo, M.A. on July 21, 2014

Background: Autism Spectrum Disorder (ASD) is usually detected in young children experiencing atypical development in the areas of communication, behavior, and social interaction. ASD is four times more common in boys than in girls. Researchers have long suspected that sex-related genetic factors may play a role in this disorder.
What’s new: On June 3, 2014, the journal Molecular Psychiatry published a study evaluating a possible link between ASD and the precursors to the male sex hormone testosterone in the amniotic fluid surrounding a developing fetus. The researchers analyzed amniotic fluid from mothers of boys born between 1993 and 1999 in a Danish cohort. In the samples from the 128 boys who later received an ASD diagnosis, the researchers found higher levels of male hormones–as well as a protein known to control hormone activity–than in the samples from the 217 controls.
Why it’s important: Using more than 19,000 amniotic fluid samples, scientists have shown a provocative link betweenelevated levels of steroid hormone and exposure in the womb to later development of ASD. However, further research is needed using different population samples to establish this link for any future clinical application.
Help me understand :
| Source(s) : |
| Tweet |
Strong Evidence for a Genetic Link to Autism
By Sharmila Banerjee-Basu, Ph.D. on July 10, 2014
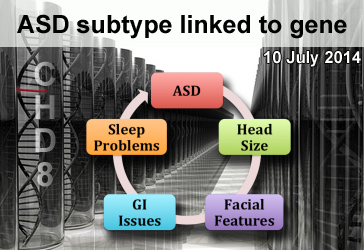
Background: The genetic landscape of Autism Spectrum Disorder (ASD) is complex. Diverse types of DNA variation—from rare mutations with large effects to common variations with small effects—are thought to contribute to the development of the disorder. Many autism-associated mutations are known, but how each one contributes to autism is not well established.
What’s new: A new study published online in the scientific journal Cell shows convincing evidence that the gene CHD8 is linked to a subtype of autism. Researchers identified eight new CHD8 mutations in 3,730 individuals with developmental delay or ASD. In contrast, the team failed to find similar CHD8 mutations in 8,792 control individuals. Including ASD individuals carrying CHD8 mutations from previous studies, the researchers further examined detailed clinical characteristics in a total of 15 individuals with CHD8 mutations. In addition to ASD, CHD8 mutation carriers have many common features, including significantly increased head size, distinct facial features, gastrointestinal (GI) issues, and sleep problems.
Why it’s important: With rapid advances in genomic technologies, a field of study is emerging where sub-types of autism can be recognized based on mutations in specific genes. Importantly, 13 out of 15 individuals with CHD8 mutations in this study had a diagnosis of ASD, indicating a strong link between CHD8 disruption and ASD onset. One can hope that genetic testing may aid in the diagnosis and treatment decision making in autism in the near future.
Help me understand :
| Source(s) : |
| Tweet |
Are Females Protected Against Autism by Their DNA?
By Eric Larsen, Ph.D. on April 9, 2014

Background: One of the hallmarks of Autism Spectrum Disorder (ASD) is its increased prevalence in males, with an estimated male-to-female ratio of four-to-one. Scientists hypothesize that this gender bias may be the result of the “female protective model.” According to this model, a greater extent of genetic damage is required to overcome an inherent protective mechanism in females. Subsequently, ASD is found more often in males, who require less genetic damage to trigger ASD onset.
What’s new: A recent report in the American Journal of Human Genetics analyzes the genetic variation present in male and female individuals with ASD. The authors report that females with ASD possess a greater number of damaging genetic variants compared to males on the spectrum. Moreover, damaging genetic variations are inherited more often from mothers, rather than fathers. Given that males and females have a different set of sex chromosomes (females have two X chromosomes, and males have an X an Y chromosome), the authors questioned if genetic changes to the X chromosome might put males at higher risk for ASD. They concluded, however, that genetic variation in the X chromosome made very little contribution to ASD.
Why it’s important: This study strengthens the case for a “female protective model” in ASD. Future research will likely seek to understand the underlying basis for this protective effect in females. Furthermore, the findings of this study may have potential implications in the interpretation of genetic screening studies in ASD cases.
Help me understand :
| Source(s) : |
| Tweet |
Shared Genetics: ASD and Schizophrenia
By Eric Larsen, Ph.D. on March 6, 2014

Background: New high-throughput sequencing technologies are providing unprecedented view of the human genome. Exome sequencing—a method targeting the protein-coding part of the genome—is increasingly used to unravel genetic underpinnings of common, complex disorders such as autism and schizophrenia.
What’s new: In the February 13, 2014, issue of Nature, two studies reported mutations identified in exomes of large cohorts of individuals with schizophrenia and a control population. The first study analyzed rare, disruptive mutations in the protein-coding regions of approximately 2,500 genes previously implicated in schizophrenia. The team evaluated DNA of 2,536 schizophrenia cases and 2,543 healthy individuals. The second study analyzed the exomes of 600 schizophrenia trios (affected individuals along with their parents). Both studies found mutations distributed across many genes; many of these genes share function at the neuronal synapse—where two neurons communicate. Of particular importance, the authors demonstrated that the schizophrenia-associated genes identified in their studies overlapped with autism-associated genes.
Why it’s important: These findings illustrate that many rare mutations contribute to schizophrenia. Furthermore, genes enriched in a number of critical cellular pathways active at the neuronal synapse are preferentially targeted by rare disruptive mutations in schizophrenia. A shared genetic architecture between schizophrenia and other neurodevelopmental disorders is becoming evident.
Help me understand :
| Source(s) : |
| Tweet |
Bioinformatics Links ASD to Stage of Fetal Development
By Sabina Muend, PhD on February 27, 2014
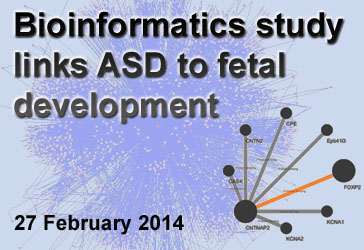
Background: Researchers have now linked several hundred genes to Autism Spectrum Disorder (ASD). Given the large number of genetic variations implicated in ASD, it is unfeasible to understand how all of these genes—and the proteins they encode—connect using experimental techniques. Therefore, researchers use bioinformatics, the process of organizing, storing, and retrieving data for analyses. One such use of bioinformatics is to make protein networks, also known as “interactomes,” which map how proteins function together in a cell.
What’s new: Two recent papers, both published in the scientific journal Cell, used bioinformatics to study the timing of brain development and the parts of the brain that are most associated with ASD. In the first analysis, a collaboration of researchers from across the United States found that 13 to 24 weeks post conception is a particularly sensitive time period based on the level of ASD-related gene products in a specific type of neuron. In the second study, University of California, Los Angeles, researchers used protein interactome mapping to link ASD with specific intracellular pathways. For instance, the interactome revealed that many of the ASD-related genes are highly expressed during synaptic development—a critical process for establishing neuron communication and brain circuitry.
Why it’s important: Based on these studies, and others like them, bioinformatics can help us understand complex disorders as well as basic cellular functioning. In the case of ASD, protein interactomes can tweeze out sensitive stages and locations of development where the impact of ASD-related genes is the greatest. Researchers hope this information could aid in the creation of finely tuned treatments.
Help me understand :
| Source(s) : |
| Tweet |
Drug Sheds Light on Underlying Cause of Autism
By Shana R. Spindler, Ph.D. on February 11, 2014

Background: With so many different genetic and environmental influences on autism, researchers are seeking a common link among risk factors. One thought is that different risk factors affect neuron function through a similar mechanism. For example, during birth there are maternal hormones that help the fetus deal with the stress of delivery and prepare the brain for post-natal development. Recent research suggests that understanding the dynamics of these hormones gives significant insight into newborn brain development.
What’s new: An animal study demonstrated that bumetanide, a commonly used diuretic drug, alters the effect of these maternal hormones. Researchers used two animal models of autism: one genetic and one drug-induced. In each model, the rodent’s progeny failed to undergo the typical brain changes in response to the labor hormone oxytocin. However, when the researchers administered bumetanide one day before delivery, the rodent’s offspring showed normal neuron function and fewer autism-like symptoms. According to the study, bumetanide enabled specific neurons to switch from an excitatory to an inhibitory state by changing chloride levels in the neuron.
Why it’s important: This study sheds light on autism’s underlying mechanism, linking both genetic and environmental risk factors to oxytocin’s effect on brain changes during birth. The finding, reported in the February 7, 2014, edition of Science magazine, supports a common mechanism for autism and paves the way for novel therapeutic strategies.
Help me understand :
| Source(s) : |
| Tweet |
Blood Test Distinguishes Those with Autism
By Shana R. Spindler, Ph.D. on January 6, 2014

Background: Today, doctors diagnose Autism Spectrum Disorder (ASD) using behavioral evaluation, limiting how early in development a doctor can detect signs of autism. For this reason, researchers are looking for autism biomarkers, measurable features that are predictive of the disorder. One line of research investigates how much genes are turned on or off in the body. Called a “gene expression profile,” this particular measurement could distinguish children with ASD.
What’s new: Using DNA chip technology, researchers at Boston Children’s Hospital and Harvard Medical Center analyzed gene expression profiles in the blood cells of 20 pairs of siblings, in which only one sibling had ASD, and 18 unrelated control individuals. According to their study, 189 genes differed in expression between the affected and unaffected siblings. Surprisingly, a small group of the unaffected siblings had gene expression profiles that more closely matched the unrelated individuals than their own sibling. The genes that differed in expression are known to play a role in cell maintenance, energy production, neural signaling, immune response, and calcium signaling pathways.
Why it’s important: This study supports the idea that gene expression profiles may help predict if a child will develop autism. This has implications for early diagnosis, or at the very least, the identification of children that may need closer monitoring of developmental progress. Additional studies with larger sample sizes are needed to verify the study’s findings.
Help me understand :
| Source(s) : |
| Tweet |
Half-Sibling Study Reveals ASD Recurrence Risk Factors
By Shana R. Spindler, Ph.D. on August 23, 2013

Background: What is the autism spectrum disorder (ASD) risk for a child who has an older sibling with ASD? Most researchers agree that a mix of genetics and environment affects risk, but they disagree on how much each factor plays a part. This makes risk prediction tricky. Previous reports indicate a wide range in ASD recurrence—when a second child has ASD.
What’s New: In a population-based study, researchers observed ASD recurrence rates in full- and half-siblings. The research team used records for all children, about 1.5 million, born in Denmark between January 1, 1980 and December 31, 2004.
The researchers report an overall recurrence risk of 7 percent. This is significantly higher than the population ASD risk of about 1.2 percent. For half-siblings sharing a father, recurrence rates were similar to the general population risk. In contrast, 2.4 percent of maternal half-siblings had ASD.
Why It’s Important: This study indicates a somewhat lower rate of recurrence than previously reported, a valuable finding for parents. A greater recurrence rate in maternal half-siblings suggests a key role for fetal environment in ASD risk. Also, the smaller recurrence risk in half-siblings, who share fewer genes, confirms a significant genetic contribution.
Help me understand :
| Source(s) : |
| Tweet |
Risperidone-Associated Weight Gain Linked to Genetics
By Shana R. Spindler, Ph.D. and Chelsea E. Toledo, M.A. on July 3, 2013
Background: Risperidone is an atypical antipsychotic drug that acts by blocking the brain’s receptors for the chemicals serotonin and dopamine. While risperidone has been approved to treat schizophrenia and bipolar disorder since 1993, in 2006 the FDA deemed it an acceptable therapy for irritability in children and adolescents with autism spectrum disorders (ASD). Unfortunately, some patients experience antipsychotic-induced weight gain while using risperidone, but little is known about the variability of this symptom.
What’s New: A new study published 25 June 2013 in the journal Translational Psychiatry suggests that risperidone-induced weight gain is influenced by genetic factors. Researchers examined common genetic variations in “energy balancing” genes in 225 patients, aged 4-17 years. These patients belonged to the National Institute of Mental Health (NIMH) Research Units on Pediatric Psychopharmacology (RUPP) Autism Network trials of risperidone for the treatment of irritability in children and adolescents with ASD. Specific genetic variants affecting Cannabinoid Receptor-1 and Leptin, two proteins known to influence feeding behavior and weight, resulted in increased risk for weight gain with the use of risperidone.
Why it’s important: Adverse side effects from pharmaceutical treatments are of important concern. Understanding the genetic and environmental risk factors for side effects can help doctors develop more personalized treatments and improve clinical outcomes.
Help me understand :
| Source(s) : |
| Tweet |
Differences in Identical Twins Reveal Autism Risk Factor
By Chelsea E. Toledo, M.A. on April 30, 2013

Background: Research has demonstrated that autism spectrum disorder (ASD) is highly heritable, suggesting a genetic basis. However, within pairs of identical twins—who have the same DNA—one sibling can have ASD, while the other doesn’t. That discordance points to the possibility of epigenetic factors, or those related to the variable expression of DNA, linked to the disorder.
What’s New: On April 23, 2013, the journal Molecular Psychiatry published a study analyzing epigenetic differences in identical twins, as related to ASD. The researchers took blood samples from 50 pairs of identical twins—with matching ASD diagnoses, mismatched ASD diagnoses, or matching typical development. They found variations in DNA methylation patterns—a form of epigenetic modification—at certain regions in the genomes between identical twins with different severities of autism. DNA methylation can be viewed as a mechanism to fine-tune gene expression. While identical twins have the same DNA sequence, their methylation patterns can fluctuate, potentially resulting in different diagnostic outcomes.
Why it’s important: This is the first large-scale study to examine the relationship between ASD and epigenetic differences in twins. The findings could point the way to treatment and prevention strategies based on patterns of DNA methylation.
Help me understand :
| Source(s) : |
| Tweet |
Genomic “Hot Spots” May Influence ASD Risk
By Eric Larsen, Ph.D. on April 9, 2013

Background: De novo mutations that spontaneously arise in sperm or eggs and are subsequently transmitted from parents to children are thought to play a critical role in human disease, including autism spectrum disorder (ASD). In order to better assess the importance of de novo mutations in the genetics of ASD, it is necessary to understand what internal and external factors influence the onset of such variation.
What’s new: In a recent report in Cell, whole-genome sequencing was performed on ten pairs on monozygotic twins concordant with ASD and their parents. Whole-genome sequencing allows for the detection of genetic variation throughout the human genome, not only in genes. Sequencing of parental samples was performed to identify de novo mutations present in both monozygotic twins.
In agreement with recent ASD sequencing studies, paternal age was found to play a significant role in genome-wide variation, with the children of older fathers tending to have a greater number of de novo mutations. Rather than being randomly distributed throughout the genome, de novo mutations were more likely to be located in genomic “hot-spots” where the intrinsic properties of the DNA within these regions contributed to increased mutability. A number of genes and genomic regions that have been previously associated with ASD, such as the NRXN1 gene and the 15q11-q13 chromosomal region, were found to have elevated de novo mutation rates.
Why it’s important: These findings provide a better understanding of the factors that are responsible for ASD-associated de novo genetic variation. As DNA sequencing and analysis technology continues to progress, cataloguing ASD-linked “hot spots” in the human genome may aid in the development of screening and/or diagnostic genetic testing for autism.
Help me understand :
| Source(s) : |
| Tweet |
Added Genetic Variants Modify Rett Syndrome Severity
By Eric Larsen, Ph.D. on March 14, 2013

Background: Why some individuals who carry similar genetic variants have different disease severity is unclear. For example, many individuals with Rett syndrome, a neurodevelopmental disorder related to autism spectrum disorder (ASD), carry mutations in the same gene, but exhibit a wide range of symptom severity which ranges from very severe to relatively mild.
What’s new: A recent article in PLoS One reported on two pairs of sisters with Rett syndrome that—despite having the same causative mutation in a gene known as MECP2—showed significant symptom variability. In each pair, one sister lacked the ability to speak or walk and displayed severe intellectual disability, while the other sister could speak and walk and displayed moderate intellectual disability. In order to identify additional factors in these patients that may affect disease severity, the researchers analyzed the protein-coding portions of the genome for additional genetic changes.
The girls with the more severe Rett syndrome symptoms preferentially carried potentially harmful genetic variants in genes associated with oxidative stress, muscle impairment, and autism/intellectual disability. On the other hand, the girls with less severe symptoms showed an enrichment of potentially harmful variants in genes associated with modulation of the immune system.
Why it’s important: The findings of this study suggest that additional genetic variation affecting specific biological processes can modify the severity of disease phenotypes in individuals with the same root genetic causes of disease. Genes identified as being responsible for modifying the overall disease severity could serve as potential therapeutic targets.
Help me understand :
| Source(s) : |
| Tweet |
Five Psychiatric Disorders May Share Common Cause
By Eric Larsen, Ph.D. and Shana R. Spindler, Ph.D. on March 7, 2013
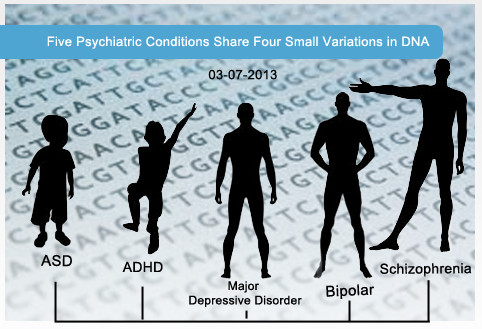
Background: While autism spectrum disorder (ASD) is generally thought of as distinct from other neuropsychiatric disorders such as schizophrenia or attention deficit-hyperactivity disorder (ADHD), the actual symptoms of ASD can show remarkable overlap with those of individuals with schizophrenia or ADHD. Likewise, genetics studies have shown that there is a significant degree of genetic overlap between ASD and other psychiatric disorders, with rare and common genetic variants affecting many of the same genes in individuals with these disorders.
What’s New: A new study published online, February 28, 2013, in The Lancet reports that patients diagnosed with one of five psychiatric disorders, including ASD, schizophrenia, bipolar disorder, ADHD, and major depressive disorder, share common variations along four spots in their DNA. While two of the locations have unknown functions, the other two are found in genes that code for subunits of ion channels that, when activated, open and allow calcium ions to enter into neurons. One of these genes, CACNA1C, is responsible for Timothy syndrome, in which as many as 80% of affected individuals are also diagnosed with ASD.
Why it’s Important: The findings of this study suggest that specific common genetic variants, in particular those variants in genes that encode for calcium channel subunits and other brain-expressed genes, can act as shared genetic risk factors for not only ASD, but other psychiatric disorders as well. These results also help to explain the sometimes-blurred boundaries between ASD and other psychiatric disorders. According to the present study, drugs that affect calcium channel signaling may offer a promising therapy for these disorders, although additional studies are required to test safety and efficacy.
Help me understand :
| Source(s) : |
| Tweet |
Females More Protected from Autism than Males
By Chelsea E. Toledo, M.A. on March 1, 2013
Background: While roughly one in 88 children has a diagnosis of autism spectrum disorder (ASD), male children are about four times more likely than female children to be diagnosed with the disorder. While researchers have suggested aspects of the female sex protect against ASD, limited studies have tested that theory.
What’s New: In the February 19 edition of Proceedings of the National Academy of Sciences, published online ahead of print, researchers evaluated the hypothesis that gender-specific traits in girls protect them from ASD. This study was designed to measure autistic traits in general population using two large twin cohorts. They found that, when a female twin ranked in or above the 90th percentile for ASD traits, her siblings were more likely to display symptoms of autism than those of males with the same degree of impairment.
Why it’s important: This study provides population-based evidence to support the theory that females are naturally protected from autism, and lends support to the genetic studies reporting that female children who are diagnosed with the disorder likely have more causal factors in their families. By identifying the protective mechanisms, researchers might gain insight into preventing or treating ASD in the future.
Help me understand :
| Source(s) : |
| Tweet |
Mitochondrial DNA Damage Linked to Autism
By Stacy W. Kish on February 14, 2013
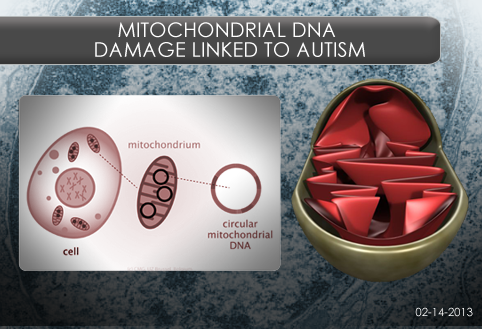
Background: Mitochondria, the powerhouses of the cell, have a set of DNA separate from the DNA in a cell’s nucleus. Some mitochondrial DNA codes for enzymes involved in energy conversion. During this conversion, reactive oxygen species (ROS) are produced that can cause damage to the cell, especially to the mitochondrial DNA. Few studies have examined mitochondrial DNA damage in children with Autism Spectrum Disorder (ASD), even though ROS production is reported to be higher in these children.
What’s new: A group of scientists at the University of California, Davis examined mitochondrial DNA (mtDNA) damage in autistic and typically developing children and their parents by direct sequencing of selected regions of their mitochondrial DNA. The participants were recruited from the CHildhood Autism Risk from Genes and Environment study (CHARGE).
The researchers found evidence of increased mtDNA damage in children with autism. The children appear to inherit mtDNA template damaged by oxidative stress from their mothers. In addition, the children may inherit a susceptibility to mtDNA deletions from their fathers, but fathers do not pass mtDNA to children.
Why it’s important: The amount of mtDNA damage in children with autism is similar to that of an older person. How environmental factors that contribute to increased mtDNA damage combine with genetic susceptibility to mtDNA deletions is an important area of investigation in the search for autism risk factors. This study provides a new line of evidence for the influence of mitochondrial DNA damage in autism.
Help me understand :
| Source(s) : |
| Tweet |
Autism Risk Linked to Differences in Infant Play
By Chelsea E. Toledo, M.A. on November 29, 2012

Background: Research has shown that infants with an autistic sibling are at an increased risk for developing Autism Spectrum Disorder (ASD). Researchers also know that the behaviors which are atypical in children with ASD--including communication and social interaction--have roots in the behaviors displayed in infancy. Lesser understood is how infants' behaviors evolve as they undergo typical development versus the emergence of ASD.
What’s New: In a report published in the print and online editions of Autism, researchers evaluated how infants at high versus low risk for ASD explored objects. They observed 31 infants--15 defined as "high risk" because they had an autistic sibling--at 6 and 9 months of age as they played with rattles. The researchers concluded that, while none of the high-risk infants went on to receive an ASD diagnosis, they did display significant differences from the low-risk group in time they spent looking at and mouthing the rattles. However, the time high- and low-risk infants spent touching the rattles with their hands was largely the same.
Why it’s important: The way infants handle objects can affect their skills later on; for instance, making sounds while chewing a rattle can help infants practice consonant sounds. Because ASD is associated with atypical language and cognition, a better understanding of the precursor behaviors for those abilities in infants teaches scientists more about how the disorder manifests in toddlers and older children.
Help me understand :
| Source(s) : |
| Tweet |
Mouse Model Reveals Critical Window of Development
By Ajay Kumar on November 20, 2012
Background: Autism Spectrum Disorder (ASD) has been linked to mutations in genes coding for proteins that function at the synapse—the location where neurons transmit electrical and chemical signals. The gene SYNGAP1 encodes one such synaptic protein. The mechanism through which synaptic dysfunction affects neural circuits and behavior is not well understood.
What’s new: Early synapse maturation can lead to irreversible cognitive and behavioral deficits in mice, according to a recent study published in the journal Cell. Researchers used a mouse model lacking the SYNGAP1 gene to investigate the role of SYNGAP1 in synapse development and, by extension, the role of synapse development in cognitive and behavior deficits. The authors reported that synapses associated with small protrusions on the receiving branches of the neuron, known as dendritic spines, mature at a faster rate in the postnatal mouse model lacking SYNGAP1. The acceleration of synapse maturation resulted in increased neural excitability and behavioral abnormalities. The researchers identified a critical developmental window, as elminating SYNGAP1 outside the window minimally impacted synapse function, and reversing the mutation in adulthood had no effect.
Why it’s important: Excitatory/Inhibitory imbalance in the brain is a striking neurophysiological feature of many neurodevelopmental disorders like ASD and Intellectual Disability (ID). This study suggests that mutations in genes that encode synaptic proteins may affect critical windows of cortical development by altering the rate of synapse development, leading to increased neural excitability and cognitive dysfunction. Further studies are needed to identify the effects of synaptic disruptions on neuronal circuit organization in the brain.
Help me understand :
| Source(s) : |
| Tweet |
Research Supports Additive Model of ASD Risk
By Stacy W. Kish on October 18, 2012

Background: Autism Spectrum Disorder (ASD) is defined by impaired social interaction and communication, as well as repetitive behaviors. With new technologies developed during the past decade, scientists began looking for genetic variations to explain ASD.
What’s new: This study examined the genomes of 2,700 families with one (simplex) or several (multiplex) autistic children. The researchers used statistical models to determine the risk of autism resulting from the contribution of both common and rare genetic variations known as single nucleotide polymorphisms (SNPs). The results suggest that a delicate interplay occurs between the additive effect of many common variants and the risk of autism, dubbed the Additive model of ASD risk. For currently unknown reasons, simplex families follow the additive model more closely than multiplex families.
Why it’s important: This work contributes to the long-standing question in the scientific community on the extent of genetic contribution to ASD. Future studies may further define the involvement of common and rare genetic variations in the risk of autism. In addition, this work may contribute to the development of diagnostic tests in the future to assess an individual’s risk of developing autism.
Help me understand :
| Source(s) : |
| Tweet |
Specific Genetic Variations May Help Predict ASD
By Shana R. Spindler, Ph.D. and Mark Ziats on October 12, 2012

Background: The diagnosis of Autism Spectrum Disorder (ASD) is currently based on clinical interviews. Doctors lack laboratory tests or other biomarkers to help support a diagnosis. Autism has a strong genetic component, but knowing which genetic variations contribute to or protect against autism is a major challenge for diagnosis.
What’s new: Scientists have developed a diagnostic test by mapping genetic variations into cellular pathways that might be affected in autism. A specific combination of 237 common genetic variations, called single nucleotide polymorphisms (SNPs), can predict an ASD diagnosis with at least 71 to 85 percent accuracy, according to a new study published 11 September in Molecular Psychiatry. The predictive accuracy, however, is only strong in those genetically similar to Central Europeans. The same SNPs predicted ASD with only 57 percent accuracy in a genetically dissimilar Chinese population. According to the authors, at least some of the genes harboring autism-linked SNPs are expressed in various brain regions implicated in ASD, and those genes are also important for cellular processes required for proper neuron functioning.
Why it’s important: An SNP profile may eventually become an important biomarker in the diagnosis of ASD. SNP testing during early infancy could allow detection of ASD before symptoms manifest, allowing for earlier therapeutic intervention, which may increase the success rate of therapy.
Help me understand :
| Source(s) : |
| Tweet |
Rett Syndrome-Linked Gene Needed for Synapse Stability
By Shana R. Spindler, Ph.D. on October 4, 2012
Background: Rett syndrome (RTT) is a genetic disorder marked by developmental regression after 6 to 18 months of age. RTT, which is almost exclusively seen in girls, is currently classified as an Autism Spectrum Disorder (ASD). Most RTT cases are caused by mutations in the MECP2 gene. Some atypical forms of RTT do exist, including those caused by variations in the CDKL5 gene. Both MECP2 and CDKL5 contribute to neuron maturation, but exactly how CDKL5 is linked to RTT symptoms is unclear.
What’s New: Researchers have uncovered a series of events that indicate an important role for CDKL5 in the stability of synapses, sites where two neurons meet to transmit electric or chemical signals. According to a new study published in the September 2012 issue of Nature Cell Biology, CDKL5 is important for the density and shape of dendritic spines, small protrusions on a neuron that harbor a synapse and receive neural signals.
Why it’s important: Studies like this help piece together how variations in different genes can lead to the same syndrome. As doctors and researchers work to develop targeted therapies for disorders within the autism spectrum, it will be important to understand how each individual’s genetic variations affect the development and function of his or her brain.
Help me understand :
| Source(s) : |
| Tweet |
The Additive Effects of CNVs on ASD Outcome
By Eric C. Larsen, Ph.D. on October 3, 2012

Background: The gains or losses of large chunks of DNA, collectively known as copy number variants (CNVs), have in recent years come under increased scrutiny as potential causative factors in human disease. While a number of CNVs at particular locations in the human genome are strongly associated with neurodevelopmental disorders such as Autism Spectrum Disorder (ASD), affected individuals who carry these CNVs display differences both in the type of disease that they have and in the severity of disease, a phenomenon known as phenotypic heterogeneity. Understanding the basis of such phenotypic heterogeneity is critical in being able to correctly interpret the results of genetic screening.
What’s new: In a recent report in the New England Journal of Medicine, researchers screened 2312 children known to carry a CNV associated with neurodevelopmental disorders and congenital anomalies for additional CNVs. The researchers determined that, in addition to the primary CNV, approximately 10% of affected children carried a second large CNV. Not only were affected children more likely to carry multiple CNVs than controls, affected children with more than one CNV tended to display more severe symptoms.
Why it’s important: These findings lend further credence to what the authors of the report refer to as the “two-hit” or second-site model, which suggests that the phenotypic heterogeneity observed in patients with ASD and other neurodevelopmental disorders results from the additive effects of multiple rare genetics variants, such as CNVs.
Help me understand :
| Source(s) : |
| Tweet |
Autism-Associated Genetic Variant Alters Brain Circuitry
By Eric Larsen, Ph.D. on September 21, 2012
Background: Understanding how genetic variation leads to changes in brain circuitry underlying social behaviour is a critical issue in autism research. A common variant in the autism-linked gene known as MET has been identified as a genetic factor that may increase both the risk and severity of ASD. The autism-linked MET variant can be found in both unaffected individuals and people with Autism Spectrum Disorder (ASD).
What’s new: Researchers in Los Angeles, CA imaged the brains of 75 children and adolescents with ASD and 87 unaffected individuals, some of whom carry the risk variant of the MET gene, to determine if the presence of this risk variant affects brain circuitry. The researchers reported in the 6 September 2012 issue of Neuron that both unaffected individuals with the MET risk variant and those with ASD who harbor the same variation in the MET gene showed noticeable changes in how the brain responded to social stimuli. The researchers also identified abnormalities in brain connectivity, both in terms of function and structure. These effects were significantly more pronounced in individuals with ASD.
Why it’s important: The results of this study offer insight into how genetic variation affects brain circuitry in children with ASD as well as unaffected individuals. The circuitry changes caused by the MET risk variant can lead to an increased risk or increased severity of ASD.
Help me understand :
| Source(s) : |
| Tweet |
More Evidence for a Common Mechanism in Autism
By Stacy W. Kish on September 20, 2012

Background: Autism has been linked to hundreds of rare genetic mutations. Core symptoms of autism often accompany single-gene syndromes, like Fragile X. However, most autism cases do not meet the clinical diagnosis for a certain syndrome. These cases are referred to as nonsyndromic. Little is known about the similarity of biological mechanisms that underlie symptoms in syndromic and nonsyndromic forms of autism. One nonsyndromic form of autism under investigation is linked to a particular gene known as neuroligin-3 (NLGN3).
What’s new: Researchers used a mouse model to examine how the absence of NLGN3 affected cerebellum connectivity. The researchers found that NLGN3 is required for the proper function of particular postsynaptic receptors (mGluRs) during the wiring and communication of neurons known as Purkinje cells (a type of neuron important for motor coordination). Interestingly, problems in mice lacking NLGN3 are similar to those seen in mice harboring abnormalities in genes associated with Fragile X Syndrome (FMRI) and tuberous sclerosis complex (TSC2).
Why it’s important: The research suggests that some nonsyndromic and syndromic autism cases share similar abnormalities in how nerve cells project and communicate. By restoring NLGN3 gene function in juvenile mice, the researchers were able to repair Purkinje cell wiring. Drugs that have shown promise treating the underlying cause of autism symptoms in Fragile X may therefore provide new therapeutic options for some nonsyndromic autism cases as well.
Help me understand :
| Source(s) : |
| Tweet |
Rare Form of Autism Reversed in Mice
By Mark N. Ziats on September 11, 2012
Background:
Many rare forms of autism result from specific variations in a patient’s DNA. The ability to sequence long stretches of DNA has dramatically improved over recent years. Researchers can now use DNA sequencing to read the genetic code of families affected with Autism Spectrum Disorder (ASD) to find mutations that may predispose children to the disease.
What’s New:
In the 6 September 2012 online edition of Science, researchers report a new genetic mutation linked to ASD in a gene that controls the metabolism of branched chain amino acids (BCAAs, a popular supplement among athletes!). Amino acids are important molecules that affect many functions in the body including brain activity. The researchers found lower levels of plasma BCAAs in individuals who carry the mutation in both copies of their chromosomes. Reasoning that amino acids are important for communication within the brain, the authors tested an amino acid supplementation diet in a mouse model that lacked the gene identified in the ASD individuals. The researchers report that neurological symptoms were reversed in the mice put on the diet.
Why it’s important:
While cases of autism caused by this specific mutation are likely to be extremely rare, this research provides some of the first evidence that the symptoms of autism are potentially reversible after their onset. This incredible finding is likely to spur further research into treatments that may improve autism symptoms in humans.
Help me understand :
| Source(s) : |
| Tweet |
Mouse Model Reveals Therapeutic Strategy
By Shana R. Spindler, Ph.D. on September 4, 2012

Background:
Dravet’s syndrome, a childhood disorder characterized by the onset of seizures within the first year of life, has symptoms common with Autism Spectrum Disorder (ASD) including—but not limited to—hyperactivity, stereotyped behaviors, inhibited social interactions, and marked cognitive deficits. Children with Dravet’s syndrome have a mutation in a string of DNA called the SCN1A gene. While most people have two copies of SCN1A, children with Dravet’s syndrome have only one functioning copy of the gene.
What’s New:
In the 22 August 2012 online edition of the journal Nature, researchers report that genetically engineered mice lacking one copy of SCN1A exhibit hyperactivity, anxiety, increased stereotyped behaviors, deficits in social interactions, and impaired cognitive function. According to the report, a specific type of neuron, called a GABAergic interneuron, requires two copies of SCN1A to hold back the transmission of electrical stimulation. When the researchers give the mice clonazepam, a type of drug known to help neurons block the spread of electric activity, the mice no longer exhibit impaired social behaviors and cognitive deficits.
Why it’s important:
Experiments with the mouse model of Dravet’s syndrome show that disruption of neuron inhibition may be one of the underlying causes of autism. Drugs that can restore the balance between inhibition and excitation of neurons are a potential therapeutic strategy for treating symptoms in some ASD-related syndromes.
Help me understand :
| Source(s) : |
| Tweet |
Older Fathers May Increase Risk of Autism
By Catherine Croft Swanwick, Ph.D. on August 24, 2012

Background: Previous epidemiological studies have reported an association between older parents and increased risk of Autism Spectrum Disorders (ASD).
What’s New: A recent study quantified the risk of ASD as parents age. Scientists studied genetic material from families whose children developed ASD or schizophrenia despite no family history of these disorders, allowing them to search for mutations which occur spontaneously, or de novo, in embryos after they are inherited.
The research team found that DNA inherited from fathers undergoes more mutation as their age at the time of conception increases. They estimate that DNA inherited from a 20-year-old father undergoes approximately 25 de novo mutations, and that this number increases by two for every additional year of the father’s age. Unlike other studies, they found no correlation between maternal age and ASD.
Why It’s Important: Hundreds of de novo mutations have been linked with ASD. This study shows how quickly the rate of de novo mutations increases as fathers age. The average age of fathers at time of conception is steadily increasing worldwide, suggesting that advanced paternal age may play a role in the rising prevalence of ASD.
Help me understand :
| Source(s) : |
| Tweet |
Autistic Behaviors May Be Connected to Slow Synapses
By Catherine Croft Swanwick, Ph.D. on August 9, 2012

Background: Hundreds of genes are linked to Autism Spectrum Disorders (ASD). Although these genes perform a wide variety of functions, most of them influence synapses. Two well-known ASD risk genes, NRXN1 and NLGN1, are best known as cell adhesion molecules which help form synapses by sticking two neurons together.
What’s New: Emerging evidence indicates new synaptic roles for NRXN1 and NLGN1. In addition to acting as cell adhesion molecules, they send signals which regulate synapse communication. When expression of NRXN1 or NLGN1 was blocked in worms or mice, their synapses communicated slowly and for longer periods of time.
Why it’s Important: This evidence suggests a new neurobiological mechanism which may underlie autistic behaviors. Next steps for scientists include discovering exactly how slower synapse communication may affect cognition.
Help me understand :
| Source(s) : |
| Tweet |
TSC1 Evidence Shows Role for Cerebellum in Autism
By Catherine Croft Swanwick, Ph.D. on July 18, 2012
Background: Scientists have long been searching for neural circuits underlying ASD. One suspected player is the cerebellum, a brain region important for balance and movement. Among the various pieces of evidence linking the cerebellum to ASD is the observation that cerebellar pathology is a common feature of individuals with Tuberous Sclerosis Complex (TSC), a genetic disorder in which many individuals also display symptoms of autism. TSC is caused by variants of the genes TSC1 and TSC2, which inhibit signaling of the mTOR pathway.
What’s New: Researchers genetically engineered mice which did not express TSC1 in their cerebellum. These mice displayed ASD-like behaviors, including abnormal social interaction and repetitive behavior/vocalizations. They also showed abnormal function of Purkinje cells, a major cell type of the cerebellum. Treatment of the mice with rapamycin, a drug which inhibits mTOR signaling, prevented the ASD-like behaviors and cerebellar dysfunction.
Why it’s Important: This evidence provides support for the role of the cerebellum in ASD. It also demonstrates roles for TSC1 in cerebellar function.
Help me understand :
| Source(s) : |
| Tweet |
SHANK Research Reveals Important Clues about Autism
By Catherine Croft Swanwick, Ph.D. on June 20, 2012

Overview: Autism has long been thought to result from abnormal development of brain connections called synapses, but new research highlights the SHANK family as key synaptic players in this spectrum of disorders.
Background: SHANK 1, 2, and 3 are “scaffolding proteins,” so called because they literally hold together synaptic components. Many of these synaptic molecules include neurotransmitter receptors that bind to glutamate to produce an excitatory signal in the brain. SHANK2 and SHANK3 identified as ASD risk genes in 2010 and 2007, respectively.
What's New:
1) Mutations of all SHANK family members have now been linked to Autism Spectrum Disorders (ASD). The May 2012 issue of the American Journal of Human Genetics reported genetic evidence gathered from 1,158 Canadian and 456 European individuals with ASD, compared with >15,000 controls. The researchers found two types of SHANK1 mutations associated with ASD in males but not females.
2) Mouse models of ASD were recently created by manipulating the SHANK2 gene. Both models, published in Nature in June 2012, showed behaviors similar to core symptoms of ASD such as abnormal social interaction, reduced communication, and repetitive grooming or jumping. These behaviors also correlated with weaker synaptic function of excitatory neurotransmitter receptors, presumably because their SHANK2 synaptic scaffolds are missing.
Why It’s Important: Together, emerging evidence from SHANK-related research is paving the way for discovery of new targeted ASD drug treatments. For example, to test for potential new ASD treatments, one of these research groups treated their ASD mouse model with drugs that activate excitatory neurotransmitter receptors. They were able to restore normal social interaction by stimulating a type of excitatory neurotransmitter receptor called the NMDA receptor, either directly with D-cycloserine, or indirectly with an activator of another type of excitatory neurotransmitter receptor called the mGluR5 receptor.
Help me understand :
| Source(s) : |
| Tweet |
Three New Papers Link Hundreds More Genes to Autism
By Catherine Croft Swanwick, Ph.D. on April 4, 2012

Background: Three studies published today in Nature demonstrate the genetic complexity of autism spectrum disorders (ASD). Each study analyzed genes of ASD families by sequencing their exomes, the sets of genes within the genome that encode proteins. This allowed them to search for single-letter DNA mutations that arise spontaneously, or de novo, and are not inherited from either parent. Together, the studies analyzed the exomes of 622 family trios (two unaffected parents and one affected child) and 250 unaffected sibling controls.
What’s New: The three research teams found hundreds of mutated genes, independently replicating six (GRIN2B, LAMC3, SCN1A, SCN2A, KATNAL2, CHD8) by screening other populations of ASD patients and controls. Additionally, they found that de novo autism-linked mutations are four times more likely to occur on the gene copies inherited from the father than from the mother. They also confirmed that genes contributed from older fathers have higher rates of de novo mutations.
Why It’s Important: Together, new estimates of autism risk genes reach as high as 1,000. Many of these autism risk genes function similarly, suggesting identification of convergent molecular networks as one approach for tackling the enormous genetic heterogeneity of autism.
Help me understand :
| Source(s) : |
| Tweet |
Both Genetics and Environment Important in Autism
By Shana R. Spindler, Ph.D. on January 22, 2012
Overview: Autism susceptibility has a significant environmental component, according to a study published in the November issue of the Archives of General Psychiatry.
Background: Researchers from across California identified twin pairs with at least one twin having autism spectrum disorder (ASD) to investigate the relative influence of genes versus environment on the development of ASD. In total, the researchers examined 192 pairs of twins, including 54 identical twins and 138 fraternal twins, one of the largest studies of its kind.
What's New: The researchers completed a statistical analysis comparing the rate of autism diagnosis for identical versus fraternal pairs. Using their analysis, they estimated that 38 percent of ASD cases are due to genetic factors, and 58 percent are due to environmental factors. However, the study’s findings were not conclusive given that the statistical analysis did not take into account genetic susceptibility to the environment.
Why it's important: According to the report’s authors, future studies that examine the association between genetics and environment will likely enhance our understanding of autism.
Help me understand :
| Source(s) : |
| Tweet |
Genetic Changes More Severe in Girls with ASD
By Shana R. Spindler, Ph.D. on January 19, 2012
Overview: Girls require greater genetic changes than boys do to develop the repetitive behaviors associated with Autism Spectrum Disorder (ASD), report researchers in the January issue of the American Journal of Medical Genetics Part B: Neuropsychiatric Genetics.
Background: Fewer females than males are diagnosed with ASD. Researchers hypothesized that girls require larger genetic variations to develop the disorder. To test their hypothesis, the researchers examined the severity of ASD symptoms in families with and without girls with ASD.
What's new: The researchers found that boys with ASD had more repetitive behaviors than girls with ASD, in general, and that boys with female siblings with ASD had more repetitive behaviors than boys without a diagnosed sister.
Why it's important: The findings suggest that girls have a higher genetic threshold for developing repetitive behaviors associated with ASD than boys do, and that having a girl with ASD may indicate that the family’s genetic variations are more extensive. The severity of social behaviors was not linked to gender, according to the study.
Help me understand :
| Source(s) : |
| Tweet |
New DNA Reader May Aid Autism Research
By Shana R. Spindler, Ph.D. on January 13, 2012
Pop News Brief: A new DNA reader can sequence an entire genome for 00—one fifth the cost incurred using competitor machines. For autism, this new technology could quicken the pace of genetic research, make available more diagnostic tools, and provide easier access to individualized treatment. Even with this novel sequencer, however, the limitations of data analysis and current genetic knowledge will likely dictate when and how the machine will be used for autism.
Help me understand :
| Source(s) : |
| Tweet |
Strategy to Treat Angelman Syndrome Tested
By Shana R. Spindler, Ph.D. on January 4, 2012
Overview: In a recent issue of Nature, researchers report a therapeutic strategy to treat Angelman syndrome, a developmental disorder related to autism.
Background: Angelman syndrome is caused by a defective version of the UBE3A protein, which helps tag other proteins for elimination from the cell. Individuals get one paternal and one maternal copy of the UBE3A gene, but through a process known as imprinting, only the maternal copy is expressed. Normally, even if the maternal copy of the gene is nonfunctional, the paternal UBE3A remains silent.
What's new: Researchers recently identified a drug, called Topotecan, that allows cells to express the paternal copy of UBE3A by inhibiting part of the cellular machinery that winds and unwinds DNA. After injecting very small amounts of Topotecan into the brains of Angelman syndrome mouse models, the researchers found that neurons from various areas of the brain began making the UBE3A protein from the paternal copy of the gene.
Why it's important: Topotecan is a promising therapeutic strategy for treating Angelman syndrome, suggest the authors. However, the researchers also stress the importance of additional studies to investigate the off-target affects of the drug.
Help me understand :
| Source(s) : |
| Tweet |
Chromosal Deletion Causes Autistic Behaviors
By Shana R. Spindler, Ph.D. on November 11, 2011
Overview: Researchers at Cold Spring Harbor Laboratory have used a technique called chromosome engineering to eliminate a small portion of chromosome 16 in mice to evaluate the DNA segment's role in autism-like symptoms.
Background: This segment of DNA, known as 16.p11.2, contains 27 genes is one of the most common genetic risk factors for being diagnosed with autism.
What's new: In the early online edition of Proceedings of the National Academy of Sciences during the week of October 3, the researchers report that mice lacking 16.p11.2 exhibit similar behaviors to children with autism, such as difficulty adapting to new environments, sleeping deficits, hyperactivity, and repetitive behaviors.
Why it's important: This study is the first to show a causative association between the 16.p11.2 deletion and autistic features.
Help me understand :
| Source(s) : |
| Tweet |
Children with Autism Have Distinct Facial Features
By Shana R. Spindler, Ph.D. on November 11, 2011
Overview: University of Missouri researchers have discovered that children with autism have distinct facial features as compared to typically developing children.
Background: The researchers used a 3-D camera system to map 17 points on the face, comparing the facial features of 64 boys with autism to the faces of 41 typically developing boys.
What's new: According to the study, children with autism have wider eyes and a broader upper face. They also have a shorter middle region of the face with a wider mouth and philtrum (the indent above the central lip). In fact, subgroups of facial characteristics aligned with particular behaviors, suggesting unique genetic or environmental risk factors shared by children with similar facial features.
Why it's important: The ability to group children who share similar risk factors may enable the delivery of tailored treatment options to each group.
Help me understand :
| Source(s) : |
| Tweet |

